Foster, C. (1999) Studies Directed Towards the Synthesis of (—)-Ebelactone-A, PhD Thesis, Cambridge University
A paper copy of the thesis is held at the University Library, Cambridge, UK.
Acknowledgements, Abstract and Abbreviations
Chapter
1: Introduction
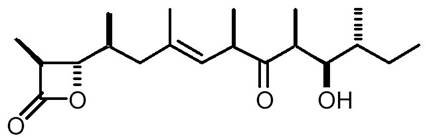
1.1 The Target Molecule 1
1.1.1 Introduction 1
1.2 ß-Lactones 3
1.2.1 Introduction 3
1.2.2 Reactions of ß-Lactones 4
1.2.2.1 Decarboxylation 4
1.2.2.2 Rearrangement 5
1.2.2.3 Nucleophilic Attack 6
1.2.2.4 Enolate Formation and Reaction 7
1.2.2.5 Conclusion 10
1.2.3 Synthesis of ß-Lactones 10
1.2.3.1 Cyclisation of ß-Halocarboxylic Acid
Salts 10
1.2.3.2 Cyclisation of ß-Hydroxycarboxylic Acids
11
1.2.3.3 [2+2] Cycloaddition Reactions 13
1.2.3.4 Other Methods 15
1.3 Paterson Synthesis of Ebelactone-a 17
1.4 General Organosilicon Chemistry 20
1.4.1 Introduction and The
Nature of the C-Si Bond 20
1.4.2 Electrophilic Attack
Directed by Silicon 22
1.4.2.1 Introduction 22
1.4.2.2 Alkylation of ß-Silyl Enolates 23
1.4.2.3 Hydroboration of Allylsilanes 23
1.4.2.4 SE2' Reaction of Allyl- and
Allenylsilanes 25
1.4.3 The
Dimethyl(phenyl)silyl Group as a Masked Hydroxyl 26
Chapter
2: Previous Work
2.1 Retrosynthetic Analysis 28
2.2 Synthesis of the Fragments 28
2.2.1 Fragment A 30
2.2.2 Fragment B 41
2.2.3 Fragment C 44
2.3 Joining the Fragments Together and Completing the Synthesis 55
2.3.1 Coupling of Fragments B
and C 55
2.3.2 Coupling of Fragment A
to the Product of Coupling of Fragments B and C 61
2.3.3 Final Transformations 64
Chapter
3: Model Coupling Work
3.1 Model Coupling of Fragment A to Fragment BC 65
3.1.1 Introduction 65
3.1.2 Vinylmetal-Aldehyde
Couplings 65
3.1.3 The Nozaki-Kishi
Reaction 68
3.1.4 Nozaki-Kishi Model
Reactions 70
3.2 Reduction of the Allylic Alcohol 80
3.2.1 Introduction 80
3.2.2 Silicon Methods 80
3.2.3 Carbamate Methodology 82
3.2.4 Other Reduction Methods 85
3.3 Conclusion 87
Chapter
4: Fragment A and the Model Coupling Reaction
4.1 Introduction 89
4.2 The Aldol Reaction 90
4.2.1 Introduction 90
4.2.2 Achiral Enolates with
Achiral Aldehydes 91
4.2.3 Achiral Enolates with
Chiral Aldehydes 93
4.2.4 Chiral Enolates with
Chiral Aldehydes 93
4.3 Oppolzer's Sultams as Chiral Auxiliaries 94
4.3.1 Introduction 94
4.3.2 Syn and Anti Methodology
95
4.3.3 The SN' Reaction 98
4.4 Synthesis of Aldehyde 155 99
4.5 The Aldol Reaction 105
4.6 Final Steps 112
Chapter
5: A New Route to Fragment A
5.1 Introduction 115
5.2 Silylcupration of Allenes 117
5.3 Nucleophilic Addition to N-ß-(Silyl)enoylsultams 121
5.4 Model Reactions on Crotonylsultam 218 123
5.4.1 Allene Methodology 123
5.4.2 Alternative Route to the
Vinylcuprate 199 127
5.5 Synthesis of N-ß-(Silyl)enoylsultam 212 131
5.6 Reaction of Vinylcuprate 199 with Sultam 212 134
5.7 Another Synthesis of Fragment A 137
Chapter
6: Experimental
6.1 Introduction 142
6.2 Compounds for Chapter 3 143
6.3 Compounds for Chapter 4 159
6.4 Compounds for Chapter 5 183
© Colin Foster, Cambridge University 1999.
Paper
© Colin Foster 2012
